Abstract

Deletion of IKZF1 (IKZF1del) is a recently described adverse prognostic factor in childhood B-lymphoblastic leukemia (B-LL). In an attempt to negate the adverse effect of IKZF1del the Ma-Spore ALL 2010 (MS2010) protocol upgraded patients to the next higher treatment risk group if they carry IKZF1del. Specifically, patients with IKZF1del are assigned to a risk group one tier higher, (i.e. SR upgraded to IR and IR upgraded to HR accordingly). Risk stratification criteria were otherwise similar between MS2003 and MS2010.
IKZF1 status was determined using SALSA MLPA P335 kit (MRC-Holland) for 410 and 275 B-LL patients treated on MS2003 and MS2010 protocols respectively. The two cohorts were similar in presenting clinical features and genetic subtypes. IKZF1del included any type of intragenic deletion or the loss of entire locus, and was compared to the "No-Del" group. IKZF1del-plus group was recently described and was defined as the co-deletion of IKZF1 and at least one of CDKN2A / CDKN2B, PAX5 or BTG1 gene in this study. The differences in 5-yr cumulative incidence of relapse (5-yr CIR) were assessed using the Gray test (univariate) or competing-risks regression based on Fine and Gray's proportional subhazards model (multivariate).
IKZF1del was found in 15.9% of entire group and 13.2% of BCR - ABL 1-negative cases. IKZF1del was overrepresented in BCR - ABL 1-positive (70.6%) and B-other (21.8%). Compared with those without IKZF1del, patients with IKZF1del were older (≥10 yrs; P<0.001), had higher WBC at diagnosis (≥50×109/L; P<0.001) as well as higher levels of Day 33 MRD (P<0.001).
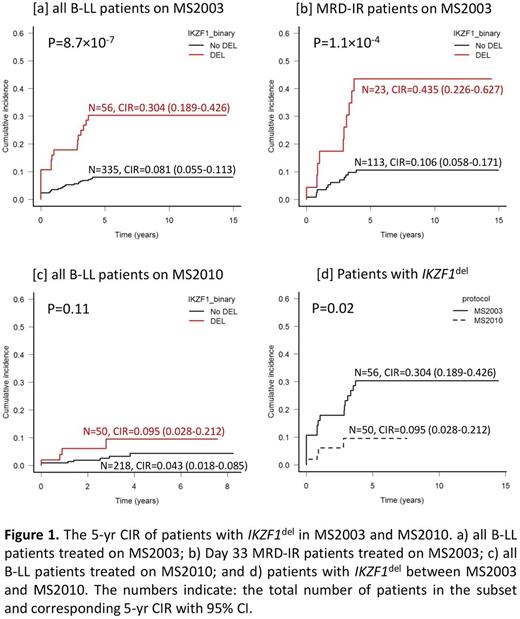
In MS2003, the overall 5-yr CIR in IKZF1del was significantly higher at 30.4% compared to 8.1% in No-Del group (P<0.001; Figure 1a). IKZF1del remained significant (P=0.043, HR with 95% CI=2.05 (1.02-4.09)) after adjusting for genetic risk, Day 8 peripheral blood response and Day 33 MRD. When stratified according to Day 33 MRD risk groups (LR<10-4; 10-4≤IR<10-2; HR≥10-2), IKZF1del conferred significantly poorer outcome only in the MRD-IR group (43.5% in IKZF1del vs. 10.6% in No-Del, P<0.001; Figure 1b) even after excluding BCR - ABL 1-positive (33.3% vs. 10.1%, P=0.009). In patients with IKZF1del-plus , 5-yr CIR was 40.0% in all patients and 60.0% in MRD-IR group, compared to respective 14.3% and 12.5% for IKZF1del-other.
In MS2010, the overall 5-yr CIR in IKZF1del improved to 9.5% compared to 4.3% in No-Del group (P=0.111; Figure 1c). This is a significant improvement when compared to MS2003 (9.5% vs. 30.4%, P=0.022; Figure 1d). The overall 5-yr CIR in patients with IKZF1del-plus also dropped from 40.0% to 17.3%. In addition, patients with IKZF1del no longer showed significantly worse outcome in any Day 33 MRD risk groups. When compared to the corresponding MRD risk group in MS2003, 5-yr CIR dropped from 7.7% to 0 in MRD-LR, from 43.5% to 18.8% in MRD-IR, and from 50.0% to 10.0% in MRD-HR. The 5-year overall survival for patients with IKZF1del was also improved from 69.6% in MS2003 to 90.6% in MS2010 (P=0.012), indicating that intensified treatment did not lead to higher incidence of toxic death.
In conclusion, our study shows that upgrading the treatment risk group for children with B-LL and IKZF1del negates the adverse outcome of IKZF1del particularly for Day 33 MRD-positive (≥10-4) patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract